Abstract
Introduction
Chemotherapy induced clonal selection and development are major contributors to disease relapse and resistance to therapy in AML. An immediate response to chemotherapy in intracellular signalling networks can be detected within minutes in vivo. (Irish J. et al 2004) We hypothesize that by analyzing the immediate intracellular chemotherapy response we might reveal which cancer clones are resistant to therapy and how they might be targeted. (Anensen N. et al Clin Cancer Res 2006), (Øyan M. et al BMC Cancer 2009)
CyTOF combines the principles of conventional flow cytometry with time-of-flight mass spectrometry to allow analyses of multiple parameters simultaneously in single cells. This dramatically increases the numbers of antibodies that can be applied and permits a simultaneous analysis of both intracellular signaling networks and phenotypic characterization of cells.
Material and methods
We have performed a 36- dimensional mass cytometry analysis (CyTOF), containing 21 surface markers (CD66b, CD16, CD45, CD11b, CD25, CD3, CD4, CD8, CD56, CD20, CD38, CD34, CD117, CD33, CD64, CD14, HLA-DR, Axl-1H12, CD90, CD7, CD123) and 15 intracellular markers (cCaspase-3, p-4EB-P1, p-Stat 5, p-Stat 3, p-Stat 1, p-p38, p- ERK ½, p-Akt, p-NFkB, p-CREB, p-S6, p-Axl, p-Rb, Cyclin B1, p-Histone H3) of over 40 de novo AML patients eligible for intensive chemotherapy. Peripheral blood was sampled at 4 hours and at 18- 24 hours after start of standard "3+7" induction therapy (Lowenberg B. et al Blood 2017). Bone marrow and peripheral blood from healthy donors were also included in the analysis.
Results
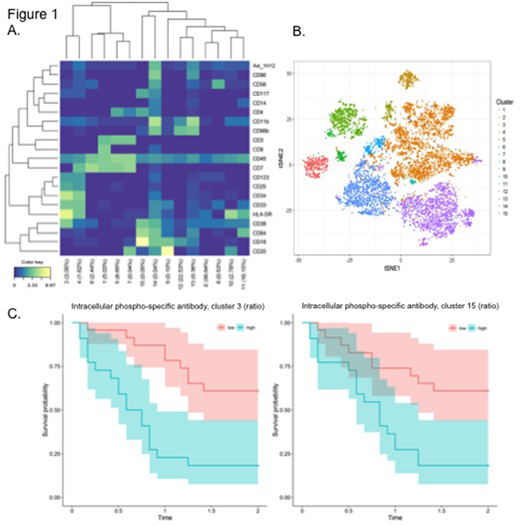
Well-characterized surface markers were used to perform an unsupervised clustering of the high dimensional single cell data from each AML patient before start of induction therapy (Figure 1A). This clustering resulted in 15 unique cell clusters throughout the patient cohort, illustrated by viSNE in Figure 2A. The heatmap in Figure 1A show the expression of surface markers in each cluster. Healthy cells in AML patients clustered together and were recognized by their characteristic phenotype, for example granulocytes in cluster 13 (CD66b+ and CD11b+), CD8 T-cells in cluster 1 (CD3+, CD7+ and CD8+) and monocytes in cluster 11 (CD45+, CD64+, CD38+ and HLA-DR+). Cluster 3 and 4 had high expression of the immature marker CD34 and thereby likely to represent blast populations.
Intracellular signaling at time of diagnosis was then compared to 24 hours after start of induction therapy in the same populations. A lasso regression analysis identified intracellular markers that significantly correlated to patient survival, based on their change in expression between pre-treatment and at 24 hours. Figure 1C show a Kaplan-Meier curve of patient survival (24 months) between patients that had high or low expression ratio of an intracellular phosphor-specific antibody in cluster 3 and 15. Both clusters have expression of CD34 and CD117 that are frequently expressed on leukemic cells. The expression ratio of this intracellular marker was significantly correlated to survival (p<0,0001). Furthermore, these CyTOF data will be correlated to RNA-seq data, mutation status and proteomic data from the same patients.
Conclusions
By applying unsupervised clustering analysis of over 40 de novo AML patients we identified leukemic cell subpopulations that had unique patterns of intracellular phospho-specific antibodies during start of induction therapy. Changes in intracellular markers during the first 24 hours of induction therapy could significantly be related to patient survival. By analyzing molecular alterations short time after chemotherapy, we aim to reveal the sub-clonal diversity and the heterogeneous responses to treatment. Identification of cancer cell subsets that are resistant to conventional chemotherapy may in the future be used to guide therapy in AML.
Gjertsen:Alden Cancer Therapy 2: Patents & Royalties: Alden Cancer Therapy II patent application in relation to CryoIT trial.; BerGenBio: Consultancy; Novartis: Consultancy; Alden Cancer Therapy 2: Membership on an entity's Board of Directors or advisory committees; Alden Cancer Therapy 2: Equity Ownership; Kinn Therapeutics: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy; Kinn Therapeutics: Equity Ownership; Boehringer Ingelheim: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal